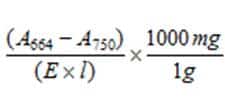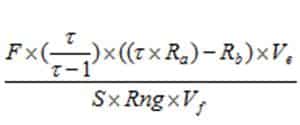Chlorophyll Determination
SUMMARY: Chlorophyll a is extracted in an acetone solution. Chlorophyll and phaeopigments are then measured fluorometrically using an acidification technique.
1. Principle
Seawater samples of a known volume are filtered (< 10 psi) onto GF/F filters. These filters are then placed into 10ml screw-top culture tubes containing 8.0ml of 90% acetone. After a period of 24 to 48 hours, the fluorescence of the samples is read on a fluorometer. Then samples are acidified to degrade the chlorophyll to phaeopigments (i.e. phaeophytin) and a second reading is taken. The readings prior to and after acidification are then used to calculate concentrations of both chlorophyll a and \’phaeopigment\’. The method used today is based on those developed by Yentsch and Menzel (1963), Holm-Hansen et al. (1965) and Lorenzen (1967). Note that concentrations of \’phaeopigments\’ are not a good measure of Chl a degradation products present in the sample since Chl b present in the sample will be measured as \’phaeopigments\’.
2. Sample Drawing
|
2.1. |
Chlorophyll bottles should be rinsed three times with sample prior to filling. The bottles are calibrated for volume, so the sample drawer must insure that air bubbles are not clinging to the sides of the bottle and it is filled completely. The sensitivity of the fluorometric method allows for sample bottles of ~50 to 250 ml. |
3. Sample Filtration
|
3.1. |
Check that the filtration funnels are well seated on the base, and be sure that the filters (Whatman GF/F) are in place. Improperly placed filters or loose funnels will result in loss of sample. The chlorophyll samples are volumetric and should sample loss occur, replace the filter with a new one and redraw the sample. |
|
3.2. |
Turn on the vacuum pump, pour the sample into the filter funnel, and open the valve. Check the vacuum pressure to see that it does not exceed 10 psi or ~500mm Hg. Generally samples are filtered in such a way as to insure that the deepest samples (i.e. those typically containing less chlorophyll) are filtered at the same manifold positions in each time. When a shallow cast is performed and a reduced number of samples is taken, it is advisable to filter them on positions typically used for those approximate depths. This reduces the potential for contaminating filter funnels used for filtering deep samples that in general contain low levels of chlorophyll. |
|
3.3. |
When a sample has finished filtering, turn off the valve; once all the samples have filtered, turn off the pump; use designated sample forceps to pick off the filter and place it in the appropriate numbered tube containing 8 mL 90% acetone. Make sure that the filter is completely submerged in the acetone. |
|
3.4. |
Cap TIGHTLY but be aware that tube tops can break off, then place the sample tubes in a rack. The sample rack is then placed in a refrigerator and the filtration time is recorded. |
4. Standardization of Fluorometer
|
4.1. |
A commercially available chlorophyll standard (e.g. Anacystis nidulans, Sigma Aldrich) should be used to calibrate the fluorometer, preferably before and after each cruise. The Chl a standard is dissolved in 100% acetone to yield approximately 0.1mg-Chl per ml solution. 1ml of this solution can then be diluted in 100ml 100% acetone and read in a spectrophotometer at 664nm. A second reading at 750nm is also recorded as a blank value to correct for sample turbidity. The remainder can be aliquotted into cryo tubes and stored in liquid N2 for future use. Chl a standards such stored are stable for years. The initial dilution is made with 100% acetone because it stores better in liquid N2 than those made with 90%. However, since 90% acetone is used for the extraction, it is also used for dilutions when generating a standard curve. |
|
4.2 |
The concentration (mg l-1) of the standard is determined by the following equation: Chl a = A664 = absorption at 664nm A750 = absorption at 750nm E = Extinction coefficient (100% acetone = 88.15, 90% acetone = 87.67) from Jefferies and Humphrey (1975) l = cuvette path length (cm) |
|
4.3. |
A series of dilutions using 90% acetone (N> 5) are then made and read, recording both Rb and Ra values. Blank values should be subtracted from the Rb and Ra prior to performing calculations. If using a fluorometer with multiple sensitivity and range settings such as a Turner model 10, then the proper blank value must be subtracted for readings taken at a given setting. |
|
4.4. |
A calibration factor (F) must be calculated for each fluorometer. It is the slope of the line resulting from plotting the fluorometer reading (x-axis) vs. chlorophyll concentration (y-axis). This line is forced through zero. An acidification coefficient (τ) is the average acid ratio (Rb/Ra) for the pure chlorophyll standards used in the calibration. |
|
4.5. |
Calculating chlorophyll and phaeopigment concentration in a sample is accomplished by using the following equations (Knap et al., 1996): Chl (µg/l) = Phaeo (µg/l) = F = Linear calibration factor (see 4.4) τ = Average acid ratio (Rb/Ra) – Note that these are actually corrected values, with the blank readings already subtracted. Ve = Volume of extract (ml) Vf = Volume of sample filtered (l) S = Sensitivity setting of fluorometer (Applicable to Turner model 10. If using a model 10AU or another fluorometer, use a value of “1”) Rng = Range setting of fluorometer (Applicable to Turner model 10. If using a model 10AU or another fluorometer, use a value of “1”) There are variations of this equation that can be used and other factors that can affect chlorophyll measurements. More detailed descriptions can be obtained in Strickland and Parsons (1968) and Holm-Hansen and Riemann (1978). Note: After a cruise, the fluorometer is calibrated again and the calibration factors and average acid ratios obtained from pre and post-cruise calibrations are averaged for final data processing. |
5. Reading Samples on the Fluorometer
|
5.1. |
The fluorometer should be allowed to warm up for approximately 1/2 hour before using it. Samples must extract in acetone for at least 24 hours prior to reading on the fluorometer and should be read before 48 hours. |
|
5.2. |
Samples must be at room temperature prior to reading. One hour before samples are to be read, they should be removed from the refrigerator and allowed to warm up in a dark place. |
|
5.3. |
A blank tube containing the same acetone batch used for the extractions should be prepared and read prior to reading samples. This blank should be read before and after every sample run and after door setting have been changed (Turner model 10 fluorometer) |
|
5.4. |
A coproporphyrin standard should be read prior to reading samples (D’Sa et al., 1997). While not used in any calculation, it is useful to monitor the performance of the fluorometer over time between calibrations. Significant changes in coproporphyrin readings may indicate a problem with the fluorometer. |
|
5.5. |
Remove the filter, shake the sample to insure that it is well mixed, and use a Kimwipe to remove fingerprints from the exterior of the tube prior to running samples. |
|
5.6. |
Read the sample and record the number (Rb). Add 100µl of 10% HCl and wait approximately 30 seconds for the number to stabilize and record the value (Ra). |
6. Equipment/Supplies
· Whatman 25mm GF/F filters (Fisher Scientific)
· Volumetric sample bottles (~130-150ml)
· Vacuum filtration apparatus with vacuum pump capable of maintaining 10 p.s.i
· Fluorometer and proper filter kit for measuring chlorophyll a/phaeophytin with acidification method (Turner model 10AU uses a 10-037R optical kit).
· Pipet (or re-pipet) capable of delivering 100µl.
· Personal protection equipment (PPE) consisting of gloves and safety glasses.
· Kimwipes or equivalent laboratory wipes.
· 10ml screw-top sample tubes (Fisher Scientific)
· Two sets of forceps (one for sample manipulation and one for replacing clean filters)
· Assorted laboratory glassware, including volumetric flasks for diluting calibration standards
7. Reagents
· Milli-Q or equivalent polished water source.
· HPLC-grade or equivalent low-fluorescing acetone. Note that volume is not conserved when preparing solution of water and acetone. The addition of 413ml Milli-Q water to 3800ml of acetone results in 4130ml of 90% acetone.
· 10% HCl solution
· Chlorophyll a (Sigma Aldrich catalog number C6144)
· Coproporphyrin III tetramethyl ester (Sigma Aldrich catalog number C7157)
8. References
· D’Sa, E.J., Lohrenz, S.E, Asper, V.L., and Walters, R.A. (1997). Time Series Measurements of Chlorophyll Fluorescence in the Oceanic Bottom Boundary Layer with a Multisensor Fiber-Optic Fluorometer. , 167: 889–896. DOI: 10.1175/1520-0426(1997)0142.0.CO;2
· Holm_Hansen, O., Lorenzen, C.J., Holms, R.W., Strickland, J.D.H. (1965). Fluorometric Determination of Chlorophyll. J. Cons.perm.int Explor. Mer. 30: 3-15.
· Holm-Hansen, O., and B. Riemann. (1978). Chlorophyll a determination: improvements in methodology. Oikos, 30: 438-447.
· Jeffery, S.W. and Humphrey, G.F. (1975). New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167: 191-194.
· Knap, A., A. Michaels, A. Close, H. Ducklow and A. Dickson (eds.). (1996). Protocols for the Joint Global Ocean Flux Study (JGOFS) Core Measurements.
JGOFS Report Nr. 19, vi+170 pp. Reprint of the IOC Manuals and Guides No. 29, UNESCO 1994.
· Lorenzen, C. J. (1967) Determination of chlorophylls and phaeopigments: spectrophotometric equations. Limnol. Oceanogr. 12: 343–346.
· Strickland J. D. H., Parsons T. R., (1968). A practical handbook of seawater analysis. Pigment analysis, Bull. Fish. Res. Bd. Canada, 167.
· Yentsch, C.S., Menzel, D.W. (1963). A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep-Sea Res. 10: 221-231